-
-
-
-
-
-
-
-
-
Why Choose
Hover to learn more Tap to learn more
UFP MedTech?
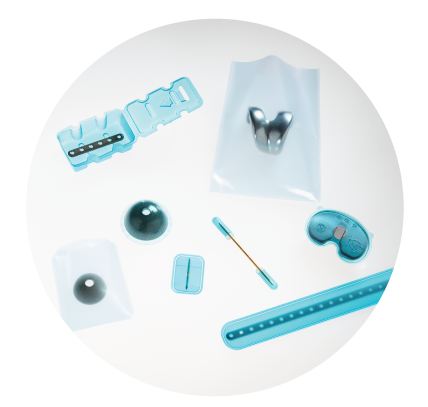
Medical Device & Sterile Packaging Manufacturing Solutions
UFP MedTech accelerates single-use and single patient devices from development through launch and manufacturing.
We are full-service, at full speed, fully on the pulse of innovation.
With decades of disposable medical device and sterile packaging manufacturing experience, we know if a great idea isn’t developed with pace, or manufactured with precision, key market milestones will be missed.
Our customer-first design and development approach is uniquely customized to each client and solution. Whether you need a partner for one phase of development, or all phases from design through manufacturing, our team of experts can guide you every step of the way.
Forging A Path Forward
Much like our focus on shaping design and engineering innovation, UFP MedTech was forged from a 60-year evolution of advancement. Discover the milestones that made us who we are today.
-
1963
1963
United Packaging Corporation incorporated; began operations as a supplier of engineered protective packaging.
-
1976
1976
Began precision molding cross-linked polyethylene foam.
-
1989
1989
Introduced compression molded protective packaging for orthopedic implants using foams specific for medical applications.
-
1994
1994
UFP invests in cleanroom production capabilities to support our growing medical customer base.
-
2001
2001
Introduced thermoplastic polyurethane (TPU) based pouches and bags to protect orthopedic implants.
-
2009
2009
First plants receive ISO 13485 certification and FDA registration.
-
2011
2011
Introduced the BioShell®, a revolutionary container system designed to protect single-use bioprocess bags during storage, handling and shipping.
-
2013
2013
Introduced FlexShield®, a product line of thermoplastic polyurethane (TPU) medical device pouches for screws, rods, plates, and other instruments.
-
2017
2017
Expanded our FlexShield® product line to include sterile barrier integrity protection for virtually any medical device.
-
2018
2018
Acquired Dielectrics, founded in 1955 and located in Chicopee MA, a leader in the design, development, and manufacture of medical devices using thermoplastic materials as well as the innovator of the Reebok Pump introduced in 1989.
-
2021
2021
Acquired Contech Medical, founded in 1987 and headquartered in Providence, RI, with partner manufacturing in Costa Rica. Contech Medical is a global leader in the design, development, and manufacture of class III medical device packaging primarily for catheters and guidewires.
-
2021
2021
Acquired DAS Medical, founded in 2010 and headquartered in Atlanta, GA, with manufacturing in the Dominican Republic. DAS Medical is a medical device contract manufacturer specializing in the design, development and production of single-use surgical equipment covers, robotic draping systems and fluid control pouches.
-
2022
2022
Acquired Advant Medical, founded in 1993 and headquartered in Galway, Ireland, with operations in Costa Rica and partner manufacturing in Mexico. Advant Medical is a developer and manufacturer of Class I, II, and III medical devices and packaging.
Passion. Pride. Precision. People.
When you partner with UFP MedTech, you get unlimited access to leading experts: medical device design, engineering, production and material specialists—each one, an extension of your team. Our core values of responsiveness and leadership drive us to better serve you and create superior outcomes with enhanced speed to market.

Chicopee, MA

Chicopee, MA

Newburyport, MA

Georgetown, MA

Newburyport, MA

Grand Rapids, MI

Chicopee, MA

Grand Rapids, MI

Grand Rapids, MI
* denotes mandatory fields.
- UFP MedTech
- © 2024 UFP MedTech
-
Corporate Headquarters: 100 Hale St.
Newburyport, MA 01950 USA - P 800-372-3172
- info@ufpmedtech.com
- UFP MedTech
- © 2024 UFP MedTech
-
Corporate Headquarters: 100 Hale St.
Newburyport, MA 01950 USA - P 800-372-3172
- info@ufpmedtech.com